Comparing A Review of Heavy Metal Uptake and Their Toxicity on Plant and Human Health
Article Information
Mario Soliman1, Shobha Potlakayala2, Daniele Millar2, Hannah Weeden2, Daniel Bogush2, Michihito Deguchi2, Sairam Rudrabhatla2*
1Burrell College of Osteopathic Medicine 3501 Arrowhead Dr, Las Cruces, NM 88001
2Penn State Harrisburg 777 West Harrisburg Pike, Middletown, PA 17057
*Corresponding author: S.airam Rudrabhatla, Penn State Harrisburg 777 West Harrisburg Pike, Middletown, PA 17057, USA
Received: 26 July 2019; Accepted: 28 August 2019; Published: 30 August 2019
Citation: Mario Soliman, Shobha Potlakayala, Daniele Millar, Hannah Weeden, Daniel Bogush, Michihito Deguchi, Sairam Rudrabhatla. Comparing A Review of Heavy Metal Uptake and Their Toxicity on Plant and Human Health. International Journal of Plant, Animal and Environmental Sciences 9 (2019): 182-189.
View / Download Pdf Share at FacebookAbstract
Heavy metal contamination in soil has become increasingly problematic in many regions around the world where anthropogenic pressures are high and is the leading cause for lost agricultural yield. A plant’s requirement of basic micronutrients for growth and development is relatively small, however natural soils contain non-essential – often toxic – elements. Despite a plant’s root cell selective membrane, much of the undesired elements found in natural soils can be detected in plant tissues. Some of the most common toxic elements to plants include Cadmium (Cd), Arsenic (As), Lead (Pb) and Mercury (Hg). Although plants do require essential micronutrients for development such as Copper (Cu), Nickel (Ni), and Zinc (Zn), excessive amounts can still be toxic for plants. Heavy metal contamination in natural soils has adverse effects on plants and even more serious effects on humans. As such, phytoremediation of contaminated soils has become increasingly important and calls for a better understanding of plant heavy metal uptake and detoxification mechanisms. In this review, we provide a concise overview plant metal uptake transporters and detoxification mechanisms. In this review, we provide a concise overview of plant metal uptake transporters and detoxification mechanisms.
Keywords
Alisk Heavy Metal Uptake Mechanisms; Phytoremediation Technology; Reactive Oxygen Species (ROS); Oxidative Stress; Metal Toxicity; Transport Proteins
Article Details
Introduction
A comprehensive investigation of the signal transduction pathway by which plants reuptake metals is essential to understanding metal accumulation and phytoremediation [1]. After metals have accumulated in the soil, the root cell binds metal ions, and subsequently to high affinity binding sites where they will be transported to plasma membrane by localized transport systems. The membrane potential of the plasma membrane, which is negative from the cytosol side, help regulate the uptake of metal cations through secondary transporters, such as channel proteins and/or proton carrier proteins [2,3]. Depending on the variety and species of the plant, there are often several pathways and mechanics through which heavy metals can be absorbed [4].
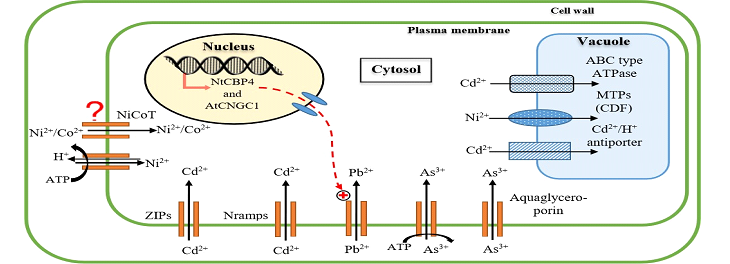
There are two well studied primary mechanisms by which plants reuptake metals through the roots: (1) apoplastic and (2) symplastic [5,6]. Due to the higher exchange capacity for cations within the apoplast, only non-cationic metal chelates can diffuse freely: this is characterized as an apoplastic movement [2]. Therefore, most of the metal ions absorbed are not soluble as they are immobile in their vascular system. The majority of highly concentrated metals absorbed by hyperaccumulator plants via symplastic movement occurs through the xylem via the stele after crossing the plasma membrane [7,8]. The plasma membrane carries a naturally negative potential which helps facilitate the cationic metals. There are three main processes that determine metal entry into the xylem (1) meta ion sequestration into root cells (2) symplastic transport into stele and (3) metal release into the xylem tissue. Once present in the xylem, metals transport through the casparian strip is mediated by membrane transport proteins, a process that requires energy to utilize active transport systems. Once the metal has been translocated inside the cell, the metal can use a cation channel in the cell membrane to move along the concentration gradient. Through various sequencing techniques, yeast mutant complementation and genomic studies on transgenic plants, several transporter gene families have been identified that suggest its aid in facilitating heavy metals; some of the identified gene families include heavy metal (or CPx-type) ATPases, ZNT (zinc-iron permease [ZIP] gene homolog) proteins, cation diffusion facilitators (CDF’s), natural resistance- associated macrophage proteins (NRAMPs), cation transporters, and the ZIP family (Figure 1) [4,7-9].
Properties, Hazards, and Uptake Mechanism of Heavy Metals in Plants
Arsenic (As)Arsenic (atomic number 33), a semi-metallic metal, is a brittle steel-gray metalloid with an atomic mass of 74.92 u, melting point of 817°C at 28 atm, vapor pressure of 1 mm Hg at 372°C, and specific gravity 5.73 kg/m3 [10]. In the soil, Arsenic (As) can combine with other elements to form organic arsenicals or inorganic arsenic compounds if combines with elements such as oxygen, chlorine, and sulfur [3]. It is important to note that As exists in the environment in several oxidation states (−3, 0, +3 and +5). Each state of the metal is absorbed through different transduction pathway [3, 10]. In natural water, As is mostly found in its inorganic forms as oxyanions of trivalent arsenite [As(III)]/ (AsO33-) or inorganic pentavalent arsenate [As(V)] or (AsO43-). Although arsenate exists in different oxidation states due to their valence configuration, arsenite (AsO33-) and arsenate (AsO43-), the pentavalent state As5+ tends to become the major contaminant in ground water.
Arsenic is one of the contaminants found in the environment which is notoriously toxic to man and other living organisms. Patients with As poisoning can experience vomiting and encephalopathy (degeneration to brain tissue/ dysfunction). Prolonged exposure can result in cardiovascular disease and inevitably cancer in major organs, such as skin, lung, liver and kidney [11]. Due to the highly toxic nature of inorganic compounds of As, phytoremediation can be an effective way to degrade soil contamination [12,13].
In most plant species, arsenate (As5+) uptake is mediated by its competition with phosphate (PO 3−) to bind the phosphate transporter; this mechanism has been studied in Oryza sativa [14] and, Brassica juncea [15] among others. The high affinity phosphate transporter system activity determines the arsenate accumulation at a concentration range that follows Michaelis-Menton kinetics [14]. In contrast to As5+, As3+ utilizes aquaglyceroporin multifunctional channels (Figure 1), a subset of the aquaporin family, for transportation. Of note, these aquaglyceroporins have been reported in several plant species [2,16].
Cadmium (Cd)Cadmium (atomic number 48), a major air and soil contaminant, is a soft bluish-white metal with an atomic mass of 112.41 u, melting point of 321.1°C, vapor pressure of 1 mm Hg at 394°C, and specific gravity of
8.65 kg/m3 [17]. Cadmium (Cd) is released into the environment in fluctuating amounts from natural and anthropogenic activities, such as forest fires and volcanic eruptions. In addition, Cd can be found in the environment weathering parent rocks, such as Mafic and ultramafic rocks. Additionally, carboniferous shale, also known as black shale, contains high amounts of Cd (up to 100 mg kg-1) that can be released in soil at toxic levels [18].
Toxic levels of Cd toxicity in plants, particularly green vegetables, can cause a decrease in biomass, diminish growth, reduce chlorophyll content, which can significantly reduce the plant’s ability to perform photosynthesis and associated chemical processes [11,18]. In addition to plant toxicity, high levels of Cd can also have major adverse effects to human health. Individuals living in areas of high levels of environmental Cd are highly susceptible to fatal respiratory, cardiovascular, renal diseases. Cadmium nephrotoxicity (toxicity of the kidneys) can lead to progressive renal tubular dysfunction that would lead to secondary osteoporosis [11,19,20]. As the global environmental and human health concern continues to increase, a Cd remediation and a better understanding of Cd uptake in plants is necessary.
Plant Cd uptake is facilitated through various mechanisms that operate using transport proteins. One mechanism in which plants uptake and transport of Cd2+ utilizes ZNT proteins; the variable histidyl regions of the ZNT proteins allow for Cd2+ binding and its subsequent transport [2]. Cadmium can also be transported via special metal transporters that are encoded by AtNramps (Natural resistance associated macrophages proteins); this mechanism has been reported in Arabidopsis to uptake high concentrations of Cd (Figure 1) [2,3].
Mercury (Hg)Mercury (atomic number 80), the only metallic element that is liquid at standard temperature and pressure (STP), is a shiny silver-white odorless liquid with an atomic mass of 200.59 u, vapor pressure of 1 mm Hg at 41.85°C, and specific gravity of 13,534 kg/m3 [3]. Mercury has the lowest melting point (-39°C) of all pure metals and has a low boiling point (357°C) making it an important component in industrial products. Similar to As and other metals, Hg can occur in the soil in various forms; Hg exists in three soluble forms in the environment. In highly oxidized soils, particularly soils with a low pH, mercury occurs as Hg0, the most reduced form, Hg22+ (mercurous ion), and Hg2+ (mercuric ion) [2,3].
Mercury has been a global environmental pollutant and concern due to its bioaccumulation ability in oceanic organisms, animals, as well as human beings. Mercury salts and organomercury compounds have been labeled by the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA) as one of the most poisonous substances in the environment. Despite being under stringent restrictions by the EPA and FDA, Hg soil toxicity is still a growing concern due to increased Hg pollution by major industries, such as mining, painting, petrochemicals, battery acids, fertilizers, and fungicidal sprays. Exposure to Hg can have significant adverse effects on plants and even more detrimental effects on humans.
Patients with Hg toxicity can present with muscle weakness, speech problems, blurry vision that can progress into life threatening diseases, such as Minamata disease, a serious neurological disorder that can further lead to paralysis and blindness [21,22]. Also, due to Hg’s ability to pass through the blood brain barrier, Hg can cause serious neurological disorders [22]. In addition to its hazardous effects on humans, Hg toxicity can effectively disrupt photosynthesis and oxidative metabolism processing in plants by interfering with electron transport reactions within the mitochondria and chloroplasts [2,21]. Additionally, high levels of Hg can significantly reduce plant water uptake as it disrupts the functionality of aquaporin channel activity (Figure 1). As Hg is highly toxic to plants and humans, even in the smallest amounts, it is critical to understand as to how it can be remediated from soils [3].
Nickel (Ni)Nickel (atomic number 28), a hard and ductile transition metal, is a silvery-white lustrous metal with an atomic mass of 58.69 u, melting point of 1,455°C, vapor pressure of 1 mm Hg at 1,65.85°C, and specific gravity of 8.908 g/cm3. Nickel (Ni) occurs in several oxidation states (-1, 0, +1, +2, +3, and +4), however Ni2+ is the most common in biological systems [23].
Similar to cadmium, Ni is released into the environment in various amounts from natural and anthropogenic sources (approximately 150,000 and 180,000 metric tonnes per year) [3]. Primary sources of Ni released into the environment are largely due to combustion of coal and oil for heat or power generation, steel and cement manufacturing, and nickel mining [3].
Depending on soil pH, Ni can exist as inorganic crystalline minerals or precipitates. Nickel toxicity has been shown to disrupt iron uptake and metabolism resulting in chlorosis, necrosis, and wilting in plants [23]. Furthermore, increased Ni levels can inhibit plant growth, photosynthesis, water balance, seed germination, as well as sugar transport [3,23]. In addition to its adverse effects in plants, Ni toxicity is highly hazardous to humans due to its detrimental effects on the heart and lungs [24]. A patient affected with Ni poisoning can present with vomiting, heart arrhythmias, and in some cases, lung cancer [24]. Nickel toxicity in soil is a great environmental and health concern and calls for an in depth understanding of the processes and mechanisms involved in nickel remediation possibly via phytoremediation.
In higher plants, proton electrochemical gradient generated by plasma H+/ATPase drives the primary active mechanism by which Ni is absorbed and transported across the plasmalemma and tonoplast of plant cells (Figure 1) [3]. Furthermore, once Ni crosses the plasma membrane, it can enter the vacuoles through cation diffusion facilitators (CDF’s) [3]. Although the specific Ni-uptake systems in higher plants has not been established, prokaryotes have two primary Ni-uptake mechanisms: Ni2+ Permeases, which belongs to the nickel/cobalt transporter (NiCoT) and NiK systems (shown in Figure 1), which belongs to the ABC transporter family [2,3]. Typically ABC transporters are a group of important membrane proteins that facilitate the active transport of ligands across biological membranes.
Lead (Pb)Lead (atomic number 82) is a bluish or silvery-grey soft and malleable heavy metal with an atomic mass of
- u, melting point of 5°C, vapor pressure of 1 mm Hg at 1740°C, and specific gravity of 11.34 kg/m3 [3]. In increasing order of abundance, Pb has four naturally occurring isotopes with atomic weights of 204, 207 u, 206 u, and 208 u. Lead occurs at oxidation states +2 and +4, however +2 is its typical state despite having four electrons in its outer valence shell [3]. Lead (Pb2+) was found to be highly toxic to humans and soil leading to increased restrictions placed in August of 2010 by the Environmental Protection Agency [25].
Accumulation of Pb in soils from car exhaust dust and other various gases from industrial sources can result in contamination of plants as well as animals that may be feeding on the plants [3]. In addition to plant contamination, high levels of lead poisoning have shown to have serious side effects on humans as this
contaminant reaches through the food chain. Patients presenting with Pb poisoning can develop progressive encephalopathy, brain diseases that alters brain function and/ or structure, and in some cases, can lead to death [11]. The serious adverse effects of toxic levels of Pb in plants, soil, and humans calls for better understanding of how plants may be used to remediate Pb as means of phytoremediation and the underlying mechanism. Particularly, plants such as industrial hemp that demonstrate a high accumulator capacity and strong resistance [26] deserve special attention.
Plants are able to uptake Pb primarily through the plant roots via passive absorption, however specific proteins have been studied and shown to facilitate Pb transport across the membrane movement [2]. Tobacco and Arabidopsis plants has been shown to regulate their ability to uptake Pb through NtCBP4 and AtCNGC1 protein expression (shown in Figure 1) [2,27].
Conclusion
Plant growth and development can be adversely affected by excessive amounts of heavy metals uptake as evident by through research so far. Natural soils containing high levels of toxic heavy metals and regions of high anthropogenic pressures results in a significant loss of agricultural yield. The alarming increase in the uptake of heavy metals Cd, As, Pb, Hg, and Ni has become problematic and therefore raises concern for human and plant health. Studies so far have been able to identify some of the key metal transporters and their respective gene families; however, additional research is required to better understand the metal specificity of transporters. In the past decade, the power of phytoremediation of contaminated soils has been widely recognized by research investigators. Additional phytoremediation studies are necessary to help identify distinctive metal transporters and mechanisms that allow certain plant species the capability to uptake heavy metals through phytoremediation technologies.
References
- Frankenberger, W.T. Environmental Chemistry of Arsenic. Marcel Dekker, New York (2002).
- Mishra Shruti and RS Dubey, Heavy Metal Uptake and Detoxification Mechanisms in Plants. International Journal of Agricultural Research 1 (2006): 122-141.
- Tangahu B, Abdullah S, Basri H, Idris M, Anur N, and Mukhlisin M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. International Journal of Chemical Engineering, (2011).
- Williams, L.E., J.K. Pittman and J.L. Hall. Emerging mechanisms for heavy metal transport in plants. Biochim. Acta 1465 (2000): 104-126.
- Rabêlo FHS, Jordão LT, Lavres J. A glimpse into the symplastic and apoplastic Cd uptake by Massai grass modulated by sulfur nutrition: Plants well-nourished with S as a strategy for phytoextraction. Plant physiology and biochemistry : PPB 121 (2017): 48-57.
- Dalir N, Khoshgoftarmanesh Symplastic and apoplastic uptake and root to shoot translocation of nickel in wheat as affected by exogenous amino acids. Journal of plant physiology 171 (2014): 531-536.
- Thakur S, Singh L, Wahid ZA, Siddiqui MF, Atnaw SM, Din Plant-driven removal of heavy metals from soil: uptake, translocation, tolerance mechanism, challenges, and future perspectives Environ Monit Assess 2016: 188.
- Saxena P, & Misra N. Remediation of heavy metal contaminated tropical land. In Sherameti, and A. Varma (Eds.) Soil Heavy Metals Soil Biology 2010: 431–477.
- Gaxiola RA, Fink GR, & Hirschi KD. Genetic manipulation of vacuolar proton pumps and transporters. Plant Physiology, (2002); 129(3), 967–973
- Mohan D, Pittman Arsenic removal from water/wastewater using adsorbents - A critical review. Journal of hazardous materials 142 (2007): 1-53.
- Brima EI. Toxic Elements in Different Medicinal Plants and the Impact on Human Health. International journal of environmental research and public health 2017:
- Bhattacharjee, and A.K. Mukherjee, 2004. Heavy metal-induced germination and early growth impairment in Amaranthus lividus L.: Implications of oxidative membrane damage. J Plant Biol 31 (2004): 1-11.
- Jha, A.B. and R.S. Arsenic exposure alters activity behaviour of key nitrogen assimilatory enzymes in growing rice plants. Plant Growth Reg 43 (2004): 259-268.
- Abedin MJ, J Feldmann and AA Meharg, Uptake kinetics of arsenic species in rice plants. Plant Physiol 128 (2002): 1120-1128.
- Pickering IJ, RC Prince, MJ George, RD Smith, GN George and DE Salt. Reduction and coordination of arsenic in Indian Plant Physiol 122 (2000): 1171-1178.
- Meharg AA and L Jardine. Arsenite transport into paddy rice (Oryza sativa) roots. New Phytol 157 (2003): 39-44.
- Rani A, Kumar A, Lal A, Pant M. Cellular mechanisms of cadmium-induced toxicity: a review. International journal of environmental health research 24 (2014): 378-399.
- Zschau T, Getty S, Gries C, Ameron Y, Zambrano A, Nash TH. Historical and current atmospheric deposition to the epilithic lichen Xanthoparmelia in Maricopa County, Arizona. Environmental pollution 125 (2003): 21-30.
- Khan MA, Khan S, Khan A, Alam M. Soil contamination with cadmium, consequences and remediation using organic amendments. The Science of the total environment 601 (2017): 1591-1605.
- Newbigging AM, Yan X, Le XC. Cadmium in soybeans and the relevance to human exposure. Journal of environmental sciences 37 (2015): 157-162.
- Clemens S, Ma JF. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annual review of plant biology 67 (2016): 489-512.
- Vahabzadeh M, Balali-Mood M. Occupational Metallic Mercury Poisoning in Gilders. The international journal of occupational and environmental medicine 7 (2016): 116-122.
- Bhalerao SA, Sharma AS, Poojari AC. Toxicity of nickel in plants. International Journal of Pure & Applied Bioscience 3 (2015): 345-355.
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, & Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary toxicology 7 (2014): 60-72.
- Environmental Protection Agency, Office of Enforcement and Compliance Assurance., United States of America, (n.d.). Consolidated Enforcement Response and Penalty Policy for the Pre-Renovation Education Rule; Renovation, Repair and Painting Rule; and Lead-Based Paint Activities Rule. United States Environmental Protection Agency.
- Girdhar, Madhuri, et al. Comparative assessment for hyperaccumulatory and phytoremediation capability of three wild weeds. 3 Biotech 4 (2014): 579-589.
- Sunkar R, Kaplan B, Bouché N, Arazi T, Dolev D, et al. Expression of a truncated tobacco NtCBP4 channel in transgenic plants and disruption of the homologous Arabidopsis CNGC1 gene confer Pb2+ tolerance. The Plant Journal 24 (2000): 533-542.